Abstracts - 5th October 2004 Pharmaceutical
Special Interest Group


Speakers From Left to Right - Front row: Anne
Kavanagh (Chair AM), Rebecca Booth, Gareth Lewis, Doug
Minick,
Back row: Chris Gilmore, Royston Copley (Chair PM), Ed
Collier, Colin Pulham. Inset Chris Hunter.
Abstracts
Hydrate or Anhydrate - That is the Question !
Rebecca Booth - [email protected]
Hydrated crystal structures are frequently found during the
development of new drugs. The discovery of a hydrated form,
either late in drug development or, worse still, when the drug is
on the market, has significant consequences. Some of the problems
associated with hydrates and the screening for hydrates will be
discussed. Two case studies, theophylline and an AZ compound,
will be used to exemplify some of the experimental techniques
which can be used to assess the kinetic and thermodynamic
stabilities of anhydrous vs hydrated crystal forms.
Crystal Structures from Solution NMR Data
Christopher A. Huntera, James McCabeb
Martin J. Packera, Andrea Spitaleria
aUniversity of Sheffield, Brook Hill, Sheffield S3
7HF, United Kingdom
bAstraZeneca Pharmaceuticals, Silk Road Business Park,
Charter Way, Macclesfield, Cheshire SK10 2NA, United Kingdom
NMR methods are widely applied for the determination of the
three-dimensional structure of molecules in solution.1
In recent years, we have developed a quantitative approach to
exploiting solution NMR complexation-induced changes in chemical
shift (CIS, Δδ ) for structure determination of
weakly bound complexes.2 This method has potential for
investigating the structures of aggregates involved in the
nucleation stages of crystallisation. 1H NMR dilution
studies of a series compounds has been carried out, and the
limiting complexation-induced changes in chemical shift were used
to determine the three-dimensional structure of the dimer element
in the initial stages of precipitation. The results show good
agreement with the X-ray crystal structures.
C.A. Hunter, M.J. Packer, Chem. Eur. J. 1999, 5,
1891-1897
C.A. Hunter, C.M.R. Low, M.J.Packer, S.E. Spey, J.G. Vinter, M.O.
Vysotsky, C. Zonta, Angew. Chem. Int. Engl. Ed. 2001, 40,
2678-2679
A. Spitaleri, C. A. Hunter, J. F. McCabe, M. J. Packer, S. L.
Cockcroft, CrystEngComm 2004, in press
Crystal Structure Analysis as an aid to Salt Selection
Studies
Gareth R. Lewis, AstraZeneca R&D Charnwood, Bakewell Road,
Loughborough, Leicestershire, LE11 5RH, UK
Remacemide was developed as a potential antagonist for
epilepsy, Parkinsonism and Huntingon's disease. In this talk the
crystal structure of remacemide and six of its salts will be
discussed, primarily focussing on an investigation of which
H-bond motifs and hydrophobic interactions recur across the
structural series.
A Crystallisation / Crystal Engineering Approach To Aid Salt
Selection - Anions
Edwin A. Collier1, Roger J. Davey1, Ronald
J. Roberts2 and Simon N. Black3
1. Colloids, Crystals and Interfaces Group, Department of
Chemical Engineering, UMIST, P.O. Box 88, Sackville Street,
Manchester, M60 1QD, UK
2. Preformulation & Biopharmaceutics, PAR&D, AstraZeneca,
Silk Road Business Park, Charter Way, Macclesfield, Cheshire,
SK10 2NA, UK
3. Process Engineering Group, PR&D, AstraZeneca, Silk Road
Business Park, Charter Way, Macclesfield, Cheshire, SK10 2NA,
UK
Salt screening is critical in the drug development process as
selection of an appropriate salt form can reduce significantly
the time to market of a new pharmaceutical entity. Salt formation
is often employed to modify the final drug product, representing
a simple chemical modification that can change to advantage the
physiochemical, formulation, biopharmaceutical and therapeutic
properties of a drug, without varying the basic chemical
structure.
This Ph.D. project has applied both classical crystallisation
techniques, along with the more modern day supramolecular synthon
approach of crystal engineering, to assist in the selection of
anions for the formation of novel pharmaceutical salts. Taking
the known structure of the hydrochloride salt as a starting
point, a further 23 salt forms of the pharmaceutical base (1R,
2S)-(-)-ephedrine have been produced. Optical microscopy
confirmed 19 of these forms were crystalline and the crystal
structures were subsequently solved. The hydrogen bonding
networks for these have been described using Graph set analysis
and the structure-property relationships, including morphology,
lattice and conformational energy, stability and solubility,
evaluated. This has been achieved using molecular modelling,
along with a wide variety of analytical techniques such as FTIR,
DSC, XRPD, TGA and DVS.
This work augments the conventional salt selection strategy
used in the industry. It has provided a starting point for a salt
screening strategy, using a theoretical basis for the selection
of anions that give the desired physiochemical properties
required for a successful new pharmaceutical material. Improved
understanding of the reasons for success and failure of the
commonly used anions to yield these new crystalline materials by
salt formation has also been realised.
High Throughput Crystallography - What to do with 1000 Powder
Patterns
Christopher Gilmore,* Gordon Barr and Wei Dong, Department of
Chemistry, University of Glasgow, Glasgow, G12 8QQ, Scotland, UK.
E-mail: [email protected]
With modern robotic systems and data collection methods, it is
quite possible to measure 1000 powder diffraction patterns in a
few days, and this is becoming commonplace in some pharmaceutical
laboratories where the search for polymorphs and salts is of
great importance. The problem arises as to what to do with such
data in particular how can it be grouped into classes when there
is no database of pure phases, and there may well be mixtures
present? We have developed two computer programs to address these
issues [1,2] that use techniques of multivariate analysis, and
classification to solve these problems. The formalism works as
follows:
1. Data are suitably pre-processed with background removal,
smoothing via wavelets, and peak searching.
2. Each of the n patterns is correlated with every other
pattern using the Pearson and Spearman coefficients to generate
an (n xn) correlation matrix.
3. This is used to generate a distance matrix which acts as a
source of classification to generate dendrograms,
multidimensional metric scaling, silhouettes, fuzzy clusters and
minimum spanning trees; these are tools that can partition the
data into clusters of related patterns. A typical dendrogram is
show below:
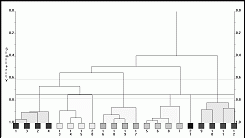
Here we have partitioned 21 patterns into 6 clusters
containing between 1 and 4 patterns. You can also represent the
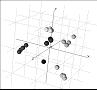
data in three dimensions using multidimensional metric scaling.
Every sphere represents a powder pattern:
[1] Gilmore, C.J., Barr, G. & Paisley, J. (2004). J.
Appl. Cryst., in press.
[2] Barr, G. Dong, W. & Gilmore, C.J., (2004). J. Appl.
Cryst., in press.
Ab Initio Vibrational Circular Dichroism and Optical
Rotation: New Tools for Determining Absolute Configurations
Douglas J. Minick, Randy D. Rutkowske; Luke A. D. Miller
Dept. of Computational, Analytical, and Structural Sciences,
GlaxoSmithKline Research, Triangle Park, North Carolina USA
X-ray crystallography remains the definitive tool for
assigning the absolute configurations of chiral drug molecules.
The number of chiral molecules under investigation by
pharmaceutical companies has risen significantly in recent years,
however, placing a growing burden on these companies to find
alternative techniques for determining this important property.
Two chiro-optical techniques, vibrational circular dichroism
(VCD) and optical rotation (OR) have emerged as techniques
capable of reliably assigning absolute stereochemistries. Yet,
these methods have remained on the sideline in most cases, often
being treated as curiosities rather than major analytical
methods. At GlaxoSmithKline (GSK), we have been performing VCD
analyses for over 3 years and have developed a robust method for
assigning configurations by this method. More recently, optical
rotation has been included in our 'chiral toolbox' as an adjunct
to VCD. While both methods remain incomplete, they have been
applied successfully in many stereochemical assignments. This
talk will include a description of the VCD method currently being
practiced at GSK, recent advances in the area of optical rotation
analysis, and conclude with an overview of our strategy for
combining these chiro-optical methods with X-ray for maximum
benefit.
High-pressure recrystallisation - a new method of screening
for polymorphs and solvates.
Colin R. Pulham, School of Chemistry, The University of
Edinburgh, King's Buildings, West Mains Road, Edinburgh, EH9 3JJ,
Scotland, UK. [email protected]
The importance of polymorphism in crystallisation processes is
widely recognised and is the subject of intense academic and
industrial interest. Although the use of high pressure has been
shown by physicists and geologists to be a powerful method for
preparing new polymorphs of metals, alloys, ceramics, and
minerals, it is only relatively recently that high pressure has
been exploited to modify intermolecular interactions in simple
molecular compounds. Thus it has been shown that new polymorphs
of simple molecular organic and inorganic compounds such as
ketones, alcohols, and mineral acids are readily obtained by
cooling the liquid compound contained within a diamond-anvil cell
under conditions of high pressure. Spectroscopic and structural
characterisation of these new polymorphs can then be performed
in situ.
Whilst this technique is ideally suited for studying compounds
that have normal melting points near or below ambient
temperature, it is less useful for compounds with higher melting
points, such as pharmaceutical compounds, pigments, or
explosives. The problem is exacerbated by the generally steep
increase in melting point associated with increasing pressure,
with the result that thermal decomposition of the compound occurs
well before the onset of melting.
We have overcome this problem by using a solvent so that
higher melting compounds are effectively recrystallised from
solution at elevated pressures, typically in the range 1-20 kbar.
This recrystallisation technique allows us to extend greatly the
range of compounds that may be studied and has been used
successfully to identify and characterise new polymorphs of
phenanthrene and acetamide. A new polymorph of piracetam have
also been identified and characterised in this way. The
high-pressure recrystallisation technique also provides a route
for the preparation and structural characterisation of new
solvates of organic compounds. For example, we have identified
and characterised a 1:1 methanol solvate of paracetamol [1], a
hydrate of parabanic acid [2], and a dihydrate of paracetamol
[3].
The technique promises to be a powerful method for the
preparation, identification, and characterisation of new
polymorphs and solvates of organic compounds such as
pharmaceuticals, pigments, and explosives. Its potential as a new
method of screening pharmaceutical compounds for polymorphism and
solvate formation is being explored and will be reported
here.
1. F.P.A. Fabbiani, D.R. Allan, A. Dawson, W. I. F. David,
P.A. McGregor, I.D.H. Oswald, S. Parsons and C. R. Pulham,
Chem. Commun., (2003), 3004.
2. F.P.A. Fabbiani, D.R. Allan, W.G.Marshall, S. Parsons, C.R.
Pulham and R.I. Smith, J. Cryst. Growth, 2004, in
press.
3. F.P.A. Fabbiani, D.R. Allan, W.I.F. David, S. Parsons, and
C.R. Pulham, CrystEngComm., 2004, in press.
|