
Left to right: Dr C.R.Lucas, Vice Chancellor, Oxford University, Professor Graham Richards, Chairman of Chemistry, Oxford, (holding the plaque) Professor Tony Ledworth, Immediate Past President, Royal Society of Chemistry, Professor Sir Tom Blundell
Since 1997 the Royal Society of Chemistry (RSC) has collaborated with the American Chemical Society of a series of International Historic Chemical Landmarks to recognise sites where important work or discoveries in the chemical sciences has been carried out, honour them with a plaque and presentation ceremony and bring them to the attention of the public and the media.
Three of these 'International Historic Chemical landmarks' have so far been awarded.
Booklets were produced to celebrate the dedication of these landmarks;
they are available from Tracey Wells at the RSC Tel: +44 (0) 20 7440 3317
email: [email protected]
website:
http://www.rsc.org/lap/publicaf/landmarks.htm
Following the success of these events, the RSC has now initiated its own National Historic Chemical Landmarks programme. Criteria have been drawn up for the Selection of these landmarks and the Society's Local Sections (35 groups of members arranged geographically in the UK and Ireland) have been invited to nominate sites of interest.
On 21 March 2001 a plaque to commemorate 'Pioneering work in platinum
research' was dedicated at the Johnson Matthey Technology
Centre, Sonning Common, Reading.
A report of the dedication of the one commemorating the work of Dorothy
Hodgkin appears later in this newsletter. A third will be dedicated in
November 2001 as part of celebrations for National Chemistry Week.
Kate Crennell
July 2001
Notes:
1. This
is a similar program to 'Blue Plaques for Physicists'
scheme described in
'Crystallography News' September 1998
and on the BCA website.
They too have a memorial to Joseph Priestley, but theirs is in
Warrington, on the Warrington Salvation Army Citadel which stands on the
site once occupied by the Warrington Academy Tutor's House where Priestley
lived from 1762 to 1767.
2. Several other memorials to scientists can
be found in the booklet describing
'The Oxford Science
Walk' by Sophie Huxley, first published in 1993 ISBN 0 9522671
0 1 cost ~£2 from the University Museum, or the Museum of the History
of Science, which was the old Ashmolean Museum first opened in 1683 and now
re-opened, following extensive excavations and renovation. The booklet is
illustrated by line drawings including one of Dorothy Hodgkin on the front
cover, most of it can be read on the museum website
http://www.mhs.ox.ac.uk
where you can find the opening times of the museum and read about other
scientific sites to see in Oxford.
Report
on the Presentation of the Royal Society of Chemistry Landmark
to Commemorate the Work of Dorothy Crowfoot Hodgkin
14 May 2001
On 14 May 2001 the Lecture Room of the University Natural History
Museum, Oxford was full of people eagerly anticipating the presentation of
the second UK Royal Society of Chemistry landmark. The proceedings were
chaired by Professor Graham Richards, Chairman of the Department of
Chemistry in the University of Oxford. The Vice-Chancellor of thestudents, Professor Sir Tom Blundell, FRS, then spoke on
"Structural Biology and Crystallography today:
the influence of Dorothy Hodgkin on current developments".
(Reported later in this issue)
In introducing the speaker, Graham Richards reminisced that he and Tom had been undergraduates together at Brasenose College, and now Tom was Dorothy's second most famous student. (the most famous being Margaret Thatcher (nee Roberts) who later abandoned chemistry for law and became Prime Minister).

Professor Tony Ledwith of the Royal Society of Chemistry then presented a memorial plaque to Professor Richards who received it on behalf of the University. Since Oxford weather is unpredictable, a virtual unveiling was projected on the screen. We saw crimson velvet curtains slowly open to reveal the plaque.

National Historic Chemical Landmark
The work of Dorothy Crowfoot Hodgkin at the University of Oxford
"In this building from 1956-1994 and at other times in the Oxford Science Area, Professor Dorothy Crowfoot Hodgkin (1910-1994) OM, FRS, Nobel Laureate, led pioneering work on the structures of antibiotics, vitamins and proteins, including penicillin, Vitamin B12 and insulin using X-ray diffraction techniques. Many methods for solving crystal structures were developed taking advantage of digital computers from the very earliest days. The work provided a basis for much of present day molecular structure driven molecular biology and medicinal chemistry."
14 May 2001
After a short time to enable us the read the inscription the image changed,
simulating a camera zooming out so that we could see the plaque was fixed to
the wall on the archway of the main entrance to the Inorganic Chemistry
Laboratory in South Parks Road.

"Structural Biology and Crystallography today:
the influence of Dorothy Hodgkin on current developments"
Professor Sir Tom Blundell FRS
Sir William Dunn Professor of Biochemistry at the University of Cambridge
Professor Blundell considered four aspects of her work:
He began by describing her early work in Cambridge with her Ph.D supervisor, J.D.Bernal, on determining the structure of protein crystals using X-rays. In 1934 she took high quality X-ray photographs of the crystalline protein pepsin, having realised that the crystals had to be kept in their mother liquor if they were to retain their order. The study of proteins in water has been immensely important over the years. The final paragraph of the paper in Nature which she and Bernal published demonstrates remarkable foresight.
"At this stage such ideas are merely speculative, but now that a crystalline protein has been made to give X-Ray photographs, it is clear that we have the means of checking them by examining the structures of all crystalline proteins, arriving at far more detailed conclusions about protein structure than previous physical or chemical methods have been able to give."
Pepsin proved to be a member of a large family of proteolytic enzymes called the aspartic proteinases which have been extensively studied since then. They have about 320 amino acids and 2 motifs of Asp-Thr-Cly at the active site. Charles Bunn was one of the first to work on rennin which was also found to be an aspartic proteinase containing this same motif; it is involved in the inhibition of angiotensin II synthesis, and may be a possible target for better drugs to reduce high blood pressure.
Pepsin has an extended deep active site and drug companies began to think about discovering drugs to fit it. In the 1980s chemistry dominated drug design, X-ray crystallography was traditionally used to model the 3D structures. Knowledge of the structure allows the design of structure based inhibitors but more recently it has proved cheaper to generate anti-hypertensive drugs using genomics.
The pepsin motif, Asp-Thr-Cly, was also identified in 1985 in the retroviral genomes of RSV and HIV, published by Toh H et al. in Nature 315, 691 (1985). Studies of the life cycle of HIV and the structure of HIV proteinase led to the production of the drug 'Indinavar', an HIV proteinase inhibitor. The 3D structure of the HIV proteinases was discovered in 1989 but later mutations were found to have occurred, so cocktails of inhibitors had to be used to improve the treatment of AIDS.
Pre-clinical Drug design now has a new paradigm since the publication of Complete Genomes in the public databases in December 2000.
(Details can be found on the Internet at http://igweb.integratedgenomics.com/GOLD/
Dorothy's interest in people led her to work on the structure of insulin,she felt she had to find the structure of insulin before a cure could be found to save children dying from early onset diabetes.
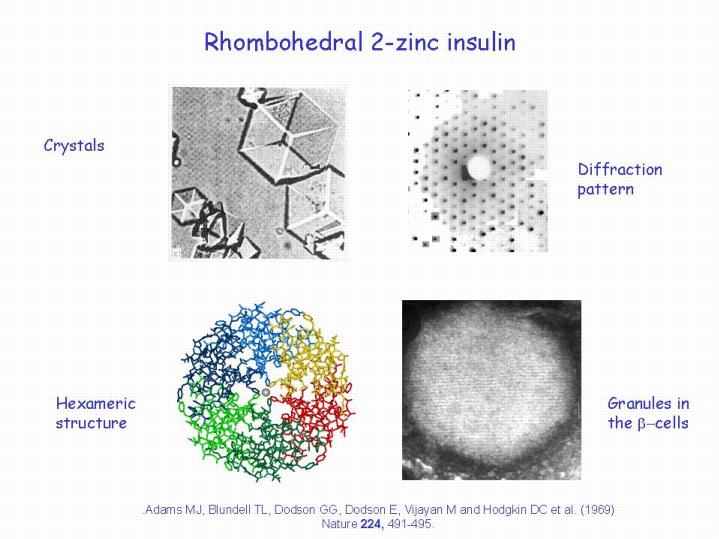
The Figure shows 4 aspects of the work on insulin,
top left, a photograph of crystals of the rhombohedral 2-zinc insulin with which the investigation began.
top right: a print of the X-Ray diffraction pattern obtained from such crystals.
lower left: the molecular structure of insulin made up of three dimers deduced from the
X-ray photograph
lower right: a photomicrograph of the granules in the beta cells of the pancreas.
Notice that all of these illustrations exhibit 3 fold symmetry.
This structure was published by Adams, M.J., Blundell, T.L, Dodson, G.G, Dodson,E, Vijau, M, and Hodgkin D.C et al. in Nature 224, 491-495 (1969). It made us think about biology in a more general way: Dorothy was not satisfied by just knowing the structure she wanted to know how the insulin carried out its function in the body. Fred Sanger showed her a model of the insulin receptor binding which she admired but was not satisfied with just knowing the structure she had to understand how the insulin behaved in the body.
She asked 'How does it work?' No one could answer that question then;
despite more recent work by Hubbard and Hendrickson in 1995 and work by
Louise Johnson on the structure of phosphorylase we still cannot answer
Dorothy's simple question.
Traditionally, laboratory X-rays were used to determine the structure of proteins and their 3D structures modelled. Today more powerful synchrotron radiation sources are mostly used. Structural biology is now being applied to aid pre-clinical drug discovery to find cures for diseases caused by stray mitogenic activity, and those involved in cell development, proliferation and differentiation. Modern computers, and new algorithms using a drug like virtual library now speed up the process of screening new drugs more effectively. Dorothy would have enjoyed using these, she always tried to use the latest equipment in her research.
Perhaps Dorothy Hodgkin's most important influence was the way she worked and interacted with people; although she collaborated with many who worked in industry, she never thought of this as collaborating with a faceless industry, but rather as working with her friends who happened to be employed by an industrial company. Her students learnt the importance of a large network of contacts to successful research.
Kate Crennell
July 2001
Note: Information about Dorothy Hodgkin can be found on the BCA web site, at http://bca.cryst.bbk.ac.uk/obits/CVS/DCH.html and there is an excellent life history at: http://www.engr.psu.edu/wep/EngCompSp98/Aclausi/HodgkinD.html
Page last updated 12 Jan 2002
 Click here to return to BCA homepage
Click here to return to BCA homepage